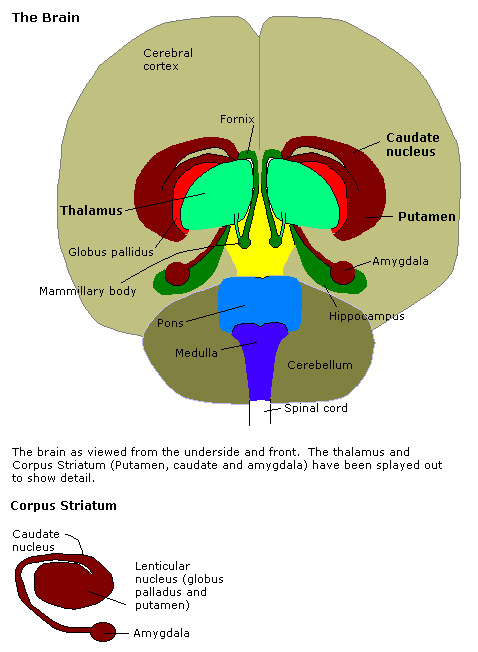
The amygdala underpins the detection and evaluation of potential threats--that is, events that undermine the safety, status, resources, autonomy, or satisfaction of individuals--but not the regulation of inhibition of threat responses. The amygdala, anatomically part of the basal ganglia, encompass several distinct nuclei, including the centromedial nucleus, the cortical nucleus, and basolateral complex, which in turn comprises the lateral, basal, and accessory basal nuclei.
As some fascinating studies show, when individuals repeat egative words to themselves--such as coffin or death--fear or anxiety might diminish. The processing of these words activates a region, called the right ventrolateral prefrontal cortex, which inhibits the amygdala (Tabibnia, Lieberman, & Craske, 2008).
Specifically, the amygdala is involved in detecting and identifying cues or stimuli that could be threatening, such as threatening facial expressions (Fitzgerald, Angstadt, Jelsone, Nathan, & Phan, 2006; Morris, Frith, Perrett, Rowland, Young, Calder, et al., 1996; Phillips, Young, Senior, Brammer, Andrews, Calder, et al., 1997; Whalen, Rauch, Etcoff, McInerney, Lee, & Jenike, 1998, Whalen, Shin, McInerney, Fischer, Wright, & Rauch, 2001). Even subliminal faces can activate the amygdala (Williams, Liddell, Kemp, Bryant, Meares, Peduto, & Gordon, 2006).
Consistent with this premise, heightened activation of the amygdala is common in individuals who experience social phobias, especially while they engage in threatening tasks such as public speaking (Lorberbaum, Kose, Johnson, Arana, Sullivan, Hamner, et al. 2004; Tillfors, Furmark, Marteinsdottir, Fischer, Pissiota, Langstrom, et al., 2001; Tillfors, Furmark, Marteinsdottir, & Fredrikson, 2002; see also Schwartz, Wright, Shin, Kagan, & Rauch, 2003).
Second, the amygdala is integral to some facets of emotional learning. That is, when the amygdala is dysfunctional, individuals do not learn to associate stimuli with fear (Critchley, Mathias, & Dolan, 2002 & Furmark, Fischer, Wik, Larsson, & Fredrikson, 1997 & LaBar, Gatenby, Gore, LeDoux, & Phelps, 1998; Morris, Ohman, & Dolan, 1998).
Specifically, information from each of the sensory systems project onto the basolateral nuclei, and the axons from this region connect to the central nucleus, which in turn projects to the hypothalamus--to affect the autonomic nervous system--and the periaqueductal gray matter--to affect behavior. Furthermore, axons from the baslateral nuclei project onto the cerebral cortex--presumably to affect subjective feelings.
Individuals experience a sense of uncertainty in many instances. Uncertainty is common in novel or unfamiliar settings, for example. In these settings, insufficient information is available to generate accurate predictions. Uncertainty tends to increase activation of the amygdala, which is typically associated with avoidance rather than approach (see Hsu, Bhatt, Adolphs, Tranel, & Camerer, 2005).
Activation of the amygdala might also increase erroneous sensitivity to hazards. In particular, after a distressing or traumatic event, such as exposure to a large spider, the amygdala increases sensitivity to related stimuli. Nevertheless, the amygdala, in essence, stores an abstracted representation of this stimulus. Hence, even a stimulus that resembles the original hazard, such as a log, is sufficient to elicit a sense of threat (for a discussion, see LeDoux, 1998).
Traditionally, researchers assumed that fear, rather than other emotions, was especially likely to activate the amygdala. Nevertheless, several arguments challenge this proposition. First, whether or not fear activates the amgdala partly depends on the methods that are used to evoke this emotion. When threatening images are presented, the amygdala tends to be activated. When other protocols are applied instead, such as memories of disturbing events, the amygdala might not be as active (Wager, Barrett, Bliss-Moreau, Lindquist, Duncan, Kober, et al., 2008). These findings imply that, perhaps, the perception of threatening stimuli, instead of merely the experience of fear, is associated with amygdala activation.
Second, as neuroimaging studies show, the amygdale is especially likely to respond to images that provoke uncertainty or ambiguity. Indeed, uncertain contexts are more likely to activate the amygdala than are stimuli that are predictable but intensely threatening (e.g., Whalen, 1998). Hence, activation of the amygdala might be evoked by unexpected, but significant, events.
Possibly consistent with this interpretation, activation of the amygdala does not correspond only to fear-or indeed only to negative emotions more generally. Processing of rewards also seems to activate this region (Murray, 2007).
Third, bilateral damage to the amygdala does not preclude the experience of fear. That is, individuals can experience negative emotions, including fear, even after the amygdalas on both sides of the brain are damaged (Anderson & Phelps, 2001, 2002).
When the amygdala is activated, individuals are more likely to remember emotional events but not as likely to remember unemotional events (see Canli et al., 2000 & Richter-Levin, 2004). Presumably, activation of the amygdala tends to shift attention to emotional objects, indicating these stimuli are especially significant. In contrast, unemotional objects are not assumed to be particularly significant when the amygdala is activated.
The amygdala also seems to impede working memory and spatial learning. Indeed, many studies show that stress in general, and activation of the amygdala in particular, disrupts these cognitive functions (Cerqueira et al., 2007 & Sandi, 2004 & Touyarot et al., 2004). That is, activation of the amygdala can inhibit circuits in the prefrontal cortex and the hippocampus.
Research indicates the amygdala is activated when individuals are exposed to topics that seem very significant--regardless of whether these issues are positive or negative. These findings coincide with the proposition that amygdala activation represents intensity, and not the valence, of emotional reactions.
To illustrate, Cunningham, Raye, and Johnson (2004) examined the brain regions that might underpin attitudes. Specifically, these authors undertook an fMRI study that was intended to ascertain the brain regions that underpin attitudes. In this study, 114 concepts were presented, such as murder, love, and abortion. For each concept, participants completed one of two tasks. They sometimes evaluated these concepts explicitly, on a scale that ranges from good to bad. Alternatively, they determined whether the concept was concrete or abstract--and thus were sometimes exposed to the item without evaluating this topic or object explicitly. In addition, fMRI was used to ascertain which regions were active during these tasks. Subsequently, participants were asked to estimate the extent to which the concept was good, bad, and intense in emotion.
Regardless of the task, the emotional intensity of concepts was correlated with activation of the left amygdala and the orbitofrontal cortex. These regions are thus sensitive to intensity, regardless of whether the concepts are evaluated explicitly, thus representing more implicit evaluations.
Borderline personality disorder is associated with elevated activation of the left amygdala, especially in response to emotional stimuli. Indeed, participants with borderline personality disorder do sometimes erroneously classify neutral faces as threatening (see Donegan, Sanislow, Blumberg, Fulbright, Lacadie, Skudlarski, et al., 2003). Accordingly, these findings indicate that excessive activation of the amygdala might underpin the excessive vigilance and undue sensitivity to interpersonal difficulties.
Recent studies indicate that neuroticism might be associated with elevated levels of amygdala activation, but only in some contexts. In one study (Haas, Omura, Constable, & Canli, 2007) a series of words superimposed on faces were presented. The participants were asked to determine whether the words were positive, negative, or neutral in their emotional tone. On some trials, the emotional tone of the word was incongruent with the emotional expression of the face. Only in these trials was amygdala activation correlated with neuroticism--at least the anxiety component.
This findings indicate that perhaps individuals who exhibit neuroticism are especially sensitive to discrepancies, such as stimuli that conflict with the context (see Eisenberger, Lieberman, & Satpute, 2005). This sensitivity might evoke arousal, which also corresponds to amygdala activation (Canli, Zhao, Brewer, Gabrieli, & Cahill, 2000).
This sensitivity of the amygdala in anxious people seems to be especially pronounced in unattended or irrelevant stimuli. To demonstrate, when neuroticism or trait anxiety is low, fearful faces that are focus of attention, but not fearful faces that are not the focus of attention, activate the amygdala considerably. In contrast, when neuroticism or trait anxiety is high, even faces that are not the focus of action will activate the amygdala considerably (Bishop et al., 2004). Indeed, even subliminal fearful faces can increase activation of the basolateral amygdala, but not dorsal amygdala, when trait anxiety is high (Etkin et al., 2004). Furthermore, neutral faces activate the amygdala in anxious people (Somerville et al., 2004): Conceivably, anxious individuals may perceive these neutral faces as threatening as well.
Besides elevated levels of amgydala activation, trait anxiety may also correlate with coupling of the amygdala and other cortical areas, including the prefrontal cortex (Bishop, 2007). To illustrate, if individuals carry the short allele of the serotonin transporter gene--an allele that coincides with trait anxiety--the feedback circuit that connects the amygdala and cingulate cortex is compromised. When this circuit is compromised, negative objects do not habituate as rapidly as usual (Pezawas et al., 2005). Furthermore, if individuals report elevated levels of anxiety, the prefrontal cortex does not tend to regulate the reactivity of the amygdala as effectively as usual (Hofmann, 2008).
Consistent with the purported relationship between amygdala activation and trait anxiety, functioning of the hypothalamus-pituitary-adrenal axis is also modulated by the amygdala. For example, glucocorticoids such as cortisol increase activity in the basolateral amygdala. This activity in the amygdala then increases the production of corticotropin-releasing-factor from the central nucleus of the amygdala, culminating in the release of ACTH from the pituitary and thus cortisol from the adrenal glands (for evidence and discussion, see Duvarci & Pare, 2007; Merali et al., 2008; Rohleder et al., 2003). Increases in cortisol are experienced as a sense of threat.
Elevated levels of cortisol--a hormone that is released by the adrenal glands under stress--has been shown to increase the size or volume of the amygdala. In particular, cortisol stimulates growth of the dentrites in neurons within the pyramidal and stellate basolateral amygdala (Fuchs et al., 2006). Synaptic connectivity of the amygdala thus increases (Vyas, A. et al., 2006). Furthermore, in humans, stress management training has been shown to diminish grey matter density in the right basolateral amygdala (Holzel et al., 2010), consistent with the proposition that decreases in cortisol reduce dentritic growth in the amygdala.
Sometimes, individuals respond impulsively to urges. They might surrender to temptations, such as eat unhealthy food when depressed or vent their resentment when angry. The amygdala does seem to underpin some impulsive acts. Nevertheless, as Brown, Manuck, Flory, and Hariri (2011) showed, activation of the ventral portion of the amygdala, which is primarily the basolateral complex, may underpin many impulsive and unsuitable acts, whereas activation of the dorsal portion of the amygdala may inhibit these acts and curb impulsivity.
In one study, participants completed a measure of impulsivity. Questions examined whether individuals tend to act without deliberation, reach decisions quickly, and fail to plan carefully. Next, they completed a task in which an angry and fearful face appeared alongside one another. Participants had to select one of these two faces, depending on whether another face, which appeared above this pair, was also angry or fearful. In addition, participants completed a similar task, but with abstract patterns instead of actual faces. As fMRI imaging showed, when the stimuli were negative faces rather than neutral patterns, activation of the amygdala increased.
Interestingly, the ventral portion of the amygdala was especially likely to be activated by the negative faces in impulsive participants. In contrast, the dorsal portion of the amygdala was especially likely to be activated by the negative faces in participants who were not impulsive (Brown, Manuck, Flory, & Hariri, 2011).
Conceivably, the dorsal amygdala may also be activated in concert with the ventrolateral prefrontal cortex, which elicits more suitable and responsible behavior. This region of the amygdala may, therefore, evoke rapid responses that nevertheless accommodate broader considerations. These responses are adaptive and may not seem impulsive. In contrast, the ventral amygdala may evoke responses to stimuli that do not integrate information from prefrontal regions.
The serotonin transport genes 5-HTT, SLC6A4 seems to correlate with activity in the amygdala. In particular, the short allele of this gene corresponds to greater levels of activation (Hariri et al., 2002; see also Bertolino et al., 2005). Furthermore, this allele is correlated with levels of trait anxiety (see Bertolino et al., 2005).
Activation of neurons in the neuroendocrine centers of the hypothalamus evokes physiological changes that culminate in cortisol secretion, which represents the stress respomse (Herman & Cullinan, 1997). The amygdala seems to be involved in the activation of these neurons, as evinced by functional relationships (Feldman & Conforti, 1981; Frankel, Jenkins, & Wright, 1978; Gallagher, Flanigin, King, & Littleton, 1987; Herman, Prewitt, & Cullinan, 1996) and anatomical considerations (Floyd, Price, Ferry, Keay, & Bandler, 2001; Rempel-Clower & Barbas, 1998).
Activation of the ventrolateral prefrontal cortex seems to reduce the activation of regions that are associated with affective experiences, such as the amygdala (e.g., Hariri, Bookheimer, & Mazziotta, 2000; Hariri, Mattay, Tessitore, Fera, & Weinberger, 2003; Lieberman, Hariri, Jarcho, Eisenberger, & Bookheimer, 2005).
In a typical study, conducted by Lieberman, Eisenberger, Crockett, Tom,Pfeifer, and Way (2007), for example, a series of emotional pictures were presented. In one condition, emotional words, such as "pain", coincided with the presentation of these pictures. In another condition, unemotional words coincided with these pictures. In the final condition, no words coincided with these pictures.
When emotional words coincided with these pictures, right ventrolateral prefrontal cortex, but not the amygdala, showed elevated levels of activation, as manifested by fMRI. In contrast, when unemotional words or no words coincided with these pictures, the amygdala showed elevated levels of activation.
Tabibnia, Lieberman, and Craske (2008) showed that conditions that activate the right ventrolateral prefrontal cortex--that is, the exposure of distressing pictures coupled with emotional words--reduced autonomic reactivity. For example, when the same distressing pictures were presented on 10 or so occasions, the skin conductance of participants diminished over time, especially when coupled with an emotional word.
Accordingly, conditions that activate the right ventrolateral prefrontal cortex also reduce the emotional intensity of events. Indeed, this region might have evolved to enable individuals to reflect upon emotional issues objectively and systematically, without the distractions of intense feelings (Tabibnia, Lieberman, & Craske, 2008).
One ongoing controversy is whether activation of the amygdala to aversive stimuli can be modulated by conscious goals, sometimes called top-down processing. In particular, when individuals are exposed to a very unpleasant object, or very pleasant, object, the amygdala tends to be activated. In addition, regions in the temporal cortex, which represent information or memories of this object, are also activated simultaneously. In the future, when individuals are exposed to the same object, activation of the temporal cortex will activate the amygdala. Furthermore, the amygdala will activate the temporal cortex. As a consequence, the activation of these regions will escalate rapidly (for reviews, see Blair & Mitchell, 2009; LeDoux, 1996).
Because these regions are activated rapidly, emotional stimuli are often more potent than other stimuli. Individuals, for example, will tend to recognize emotional terms, like "murder", more rapidly than unemotional terms, like "table" (e.g., Lorenz & Newman, 2002).
Nevertheless, some evidence indicates that individuals can override this tendency. That is, a conscious effort to orient attention towards another object can nullify this bias towards emotional stimuli. Specifically, when individuals attempt to direct their attention to some object, amygdala activation in response to fearful expressions is curbed ( e.g., Bishop, Jenkins, & Lawrence 2007). These findings indicate the conscious goals can temper the amygdala reaction to emotional stimuli.
A variety of other studies, however, challenge this assumption. That is, some research indicates that amgydala activation does not diminish, and might even increase, when attention is directed to other objects (e.g., Williams, McGlone, Abbott, & Mattingley, 2005). Furthermore, when attention is distracted by other tasks, the amygdala often responds to a broader range of emotional cues, although activation of the insular cortex diminishes (Anderson, Christoff, Panitz, De Rosa, & Gabrieli, 2003).
Anderson, A. K., Christoff, K., Panitz, D. De Rosa, E., & Gabrieli, J. D. (2003). Neural correlates of the automatic processing of threat facial signals. Journal of Neuroscience 23, 5627-5633.
Anderson, A. K., & Phelps, E. A. (2001). Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature, 411(6835), 305-309. .
Anderson, A. K., & Phelps, E. A. (2002). Is the human amygdala critical for the subjective experience of emotion? Evidence of intact dispositional affect in patients with amygdale lesions. Journal of Cognitive Neuroscience, 14, 709-720.
Banks, S. J., Eddy, K. T., Angstadt, M., Nathan, P. J., & Phan, K. L. (2007). Amygdala-frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience, 2, 303-312.
Bertolino, A. et al. (2005). Variation of human amygdala response during threatening stimuli as a function of 5-HTTLPR genotype and personality style, Biological Psychiatry, 57, 1517-1525.
Bishop, S. J., et al. (2004). State anxiety modulation of the amygdala response to unattended threat-related stimuli Journal of Neuroscience, 24, 10364-10368.
Bishop, S. J. (2007). Neurocognitive mechanisms of anxiety: an integrative account. Trends in Cognitive. Science, 11, 307-316.
Bishop, S.J., Jenkins, R., & Lawrence, A.D. (2007). Neural processing of threat: Effects of anxiety are gated by perceptual capacity limits Cerebral Cortex, 17, 1595-1603.
Blair, R. J. R., & Mitchell, D. G. V. (2009). Psychopathy, attention and emotion. Psychological Medicine, 39, 543-555.
Brown, S. M., Manuck, S. B., Flory, J. D., & Hariri, A. R. (2011). Neural basis of individual differences in impulsivity: Contributions of corticolimbic circuits for behavioral arousal and control. Emotion, 6, 239-245. doi: 10.1037/1528-3542.6.2.239
Canli, T., Zhao, Z., Brewer, J., Gabrieli, J. D. E., & Cahill, L. (2000). Event-related activation in the human amygdala associates with later memory for individual emotional experience. Journal of Neuroscience, 20 RC99, 1-5
Cerqueira, J. J. et al. (2007). The prefrontal cortex as a key target of the maladaptive response to stress, Journal of Neuroscience, 27, 2781-2787.
Critchley, H. D., Mathias, C. J., & Dolan, R. J. (2002). Fear conditioning in humans: The influence of awareness and autonomic arousal on functional neuroanatomy. Neuron, 33, 653-663.
Donegan, N. H., Sanislow, C. A., Blumberg, H. P., Fulbright, R. K., Lacadie, C., Skudlarski, P., et al. (2003). Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation. Biological Psychiatry, 54, 1284-1293.
Duvarci, S., & Pare, D. (2007). Glucocorticoids enhance the excitability of principal basolateral amygdala neurons. Journal of Neuroscience, 27, 4482-4491.
Eisenberger, N. I., Lieberman, M. D., & Satpute, A. B. (2005). Personality from a controlled processing perspective: An fMRI study of neuroticism, extraversion, and self-consciousness. Cognitive Affective Behavioral Neuroscience, 5, 169-181.
Etkin, A. et al. (2004). Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron, 44, 1043-1055.
Feldman, S., & Conforti, N. (1981). Effects of hypothalamic deafferentations on adrenocortical responses in the rat following hippocampal stimulation. Experimental Brain Research, 44, 232-234.
Fitzgerald, D. A., Angstadt, M., Jelsone, L. M., Nathan, P. J., & Phan, K. L. (2006). Beyond threat: Amygdala reactivity across multiple expressions of facial affect. Neuroimage, 30, 1441-1448.
Floyd, N. S., Price, J. L., Ferry, A. T., Keay, K. A., & Bandler, R. (2001). Orbitomedial prefrontal cortical projections to hypothalamus in the rat. Journal of Comparative Neurology, 432, 307-328.
Frankel, R. J., Jenkins, J. S., & Wright, J. J. (1978). Pituitary-adrenal response to stimulation of the limbic system and lateral hypothalamus in the rhesus monkey (Macacca mulatta). Acta Endocrinologica, 88, 209-216.
Fuchs, E. et al. (2006). Remodeling of neuronal networks by stress. Frontiers of Bioscience, 11, 27-2746-2758.
Furmark, T., Fischer, H., Wik, G., Larsson, M., & Fredrikson, M. (1997). The amygdala and individual differences in human fear conditioning. Neuroreport, 8, 3957-3960.
Gallagher, B. B., Flanigin, H. F., King, D. W., & Littleton, W. H. (1987). The effect of electrical stimulation of medial temporal lobe structures in epileptic patients upon ACTH, prolactin, and growth hormone. Neurology, 37, 299-303.
Ghashghaei, H. T., Hilgetag, C. C., & Barbas, H. (2007). Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage, 34, 905-923.
Haas, B. W., Omura, K., Constable, R. T., & Canli, T. (2007). Emotional conflict and neuroticism: Personality-dependent activation in the amygdala and subgenual anterior cingulate. Behavioral Neuroscience, 121, 249-256.
Hariri, A. R. et al. (2002). Serotonin transporter genetic variation and the response of the human amygdale. Science, 297, 400-403.
Hariri, A. R., Bookheimer, S. Y., & Mazziotta, J. (2000). Modulating emotional responses: Effects of a neocortical network on the limbic system. Neuroreport, 17, 43-48.
Hariri, A. R., Mattay, V. S., Tessitore, A., Fera, F., & Weinberger, D. R. (2003). Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry, 53, 494-501
Heinz, A., Braus, D. F., Smolka, M. N., Wrase, J., Puls, I., Hermann, D., et al. (2005). Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nature Neuroscience, 8, 20-21.
Herman, J. P., & Cullinan, W. E. (1997). Neurocircuitry of stress: Central control of the hypothalamo-pituitary-adrenocortical axis. Trends in Neurosciences, 20, 78-84.
Herman, J. P., Prewitt, C. M., & Cullinan, W. E. (1996). Neuronal circuit regulation of the hypothalomo-pituitary-adrenocortical stress axis. Critical Reviews in Neurobiology, 10, 371-394.
Hofmann, S. G. (2008). Cognitive processes during fear acquisition and extinction in animals and humans: Implications for exposure therapy of anxiety disorders. Clinical Psychological Review, 28, 199-210.
Holzel, B. K., et al. (2010). Stress reduction correlates with structural changes in the amygdala. Social Cognitive and Affective Neuroscience, 5, 11-17.
Hsu, M., Bhatt, M., Adolphs, R., Tranel, D., & Camerer, C. F. (2005). Neural system responding to degrees of uncertainty in human decision-making. Science, 310, 1681-1683.
Johnstone, T., Somerville, L. H., Alexander, A. L., Oakes, T. R., Davidson, R. J., Kalin, N. H., et al. (2005). Stability of amygdala BOLD response to fearful faces over multiple scan sessions. Neuroimage, 25, 1112-1123.
LaBar, K. S., Gatenby, J. C., Gore, J. C., LeDoux, J. E., & Phelps, E. A. (1998). Human amygdala activation during conditioned fear acquisition and extinction: A mixed-trial fMRI study. Neuron, 20, 937-945.
LeDoux, J. (1996). The emotional brain. Simon & Schuster: New York.
LeDoux, J. (1998). The emotional brain: The mysterious underpinnings of emotional life. New York: Simon and Schuster.
Lieberman, M. D., Eisenberger, N. I., Crockett, M. J., Tom, S. M., Pfeifer, J. H., & Way, B. M. (2007). Putting feelings into words: Affect labeling disrupts amygdala activity to affective stimuli. Psychological Science, 18, 421-428.
Lieberman, M. D., Hariri, A., Jarcho, J. M., Eisenberger, N. I., & Bookheimer, S. Y. (2005). An fMRI investigation of race-related amygdala activity in African-American and Caucasian-American individuals. Nature Neuroscience, 8, 720-722.
Lorberbaum, J. P., Kose, S., Johnson, M. R., Arana, G. W., Sullivan, L. K., Hamner, M. B., et al. (2004). Neural correlates of speech anticipatory anxiety in generalized social phobia. Neuroreport, 15, 2701-2705.
Lorenz, A. R., & Newman, J. P. (2002). Deficient response modulation and emotion processing in low-anxious Caucasian psychopathic offenders: Results from a lexical decision task. Emotion 2, 91-104.
Merali, Z. et al. (2008). Effects of corticosterone on corticotrophin-releasing hormone and gastrin-releasing peptide release in response to an aversive stimulus in two regions of the forebrain (central nucleus of the amygdala and prefrontal cortex). European Journal of Neuroscience, 28, 165-172.
Mervaala, E., Fohr, J., Kononen, M., Valkonen-Korhonen, M., Vainio, P., Partanen, K., et al. (2000). Quanitative MRI of the hippocampus and amygdala in severe depression. Psychological Medicine, 30, 117-125.
Morris, J. S., Frith, C. D., Perrett, D. I., Rowland, D., Young, A. W., Calder, A. J., et al. (1996, October). A differential neural response in the human amygdala to fearful and happy facial expressions. Nature,383, 812-815.
Morris, J. S., Ohman, A., & Dolan, R. J. (1998, June). Conscious and unconscious emotional learning in the human amygdala. Nature, 393, 467-470.
Murray, E. (2007). The amygdala, reward and emotion. Trends in Cognitive Sciences, 11, 489-497.
Ochsner, K. N., Bunge, S. A., Gross, J. J., & Gabrieli, J. D. E. (2002). Rethinking feelings: An fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience, 14, 1215-1229.
Pezawas, L. et al. (2005). 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nature Neuroscience, 8, 828-834.
Phillips, M. L., Young, A. W., Senior, C., Brammer, M., Andrews, C., Calder, A. J., et al. (1997, October). A specific neural substrate for perceiving facial expressions of disgust. Nature, 389, 495-498.
Rempel-Clower, N. L., & Barbas, H. (1998). Topographic organization of connections between the hypothalamus and prefrontal cortex in the rhesus monkey. Journal of Comparative Neurology, 398, 393-419.
Rohleder, N. et al. (2003). Glucocorticoid sensitivity in humans-interindividual differences and acute stress effects. Stress, 6, 207-222.
Rosso, M., Cintron, C. M., Steingard, R. J., Renshaw, P. F., Young, A. D., & Yurgelun- Tood, D. A. (2005). Amygdala and hippocampus volumes in pediatric major depression. Biological Psychiatry, 57, 21- 26.
Sandi, C. (2004). Stress, cognitive impairment and cell adhesion molecules. Nature Reviews Neuroscience, 5, 917-930.
Sandi, C., & Richter-Levin, G. (2009). From high anxiety trait to depression: A neurocognitive hypothesis. Trends in Neurosciences, 32, 312-320. doi:10.1016/j.tins.2009.02.004
Schwartz, C. E., Wright, C. I., Shin, L. M., Kagan, J., & Rauch, S. L. (2003, April). Inhibited and uninhibited infants "grown up": Adult amygdalar response to novelty. Science, 300, 1952-1953.
Somerville, L. H. et al. (2004). Human amygdala responses during presentation of happy and neutral faces: Correlations with state anxiety. Biological Psychiatry, 55, 897-903.
Tabibnia, G., & Lieberman, M. D., & Craske, M. G. (2008). The lasting effects of words on feelings: Words may facilitate exposure effects to threatening images. Emotion, 8, 307-317.
Terbatz van Elst, L.,Woermann, F., Lemieux, L., & Trimble, M. R. (2000). Increased amygdala volumes in female and depressed humans. A quantitative magnetic resonance imaging study. Neuroscience Letters, 281, 103- 106.
Tillfors, M., Furmark, T., Marteinsdottir, I., Fischer, H., Pissiota, A., Langstrom, B., et al. (2001). Cerebral blood flow in subjects with social phobia during stressful speaking tasks: A PET study. American Journal of Psychiatry, 158, 1220-1226.
Tillfors, M., Furmark, T., Marteinsdottir, I., & Fredrikson, M. (2002). Cerebral blood flow during anticipation of public speaking in social phobia: A PET study. Biological Psychiatry, 52, 1113-1119.
Touyarot, K. et al. (2004). Spatial learning impairment induced by chronic stress is related to individual differences in novelty reactivity: search for neurobiological correlates. Psychoneuroendocrinology, 29, 290-305.
Vyas, A. et al. (2006). Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience, 143, 387-393.
Weniger, G., Lange, C., & Irle, E. (2006). Abnormal size of the amygdala predicts impaired emotional memory in major depressive disorder. Journal of Affective Disorders, 94, 219- 229.
Wager, T. D., Barrett, L. F., Bliss-Moreau, E., Lindquist, K., Duncan, S., Kober, H., et al. (2008). The neuroimaging of emotion. In M. Lewis, J. M. Haviland-Jones, & L. F. Barrett (Eds.), Handbook of emotions (pp. 249-271). New York: Guilford Press. .
Whalen, P. J. (1998). Fear, vigilance, and ambiguity: Initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science, 7, 177-188.
Whalen, P. J., Rauch, S. L., Etcoff, N. L., McInerney, S. C., Lee, M. B., & Jenike, M. A. (1998). Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience, 18, 411-418.
Whalen, P. J., Shin, L. M., McInerney, S. C., Fischer, H., Wright, C. I., & Rauch, S. L. (2001). A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion, 1, 70-83.
Williams, L. M., Liddell, B. J., Kemp, A. H., Bryant, R. A., Meares, R. A., Peduto, A. S., & Gordon, E. (2006). Amygdala-prefrontal dissociation of subliminal and supraliminal fear. Human Brain Mapping, 27, 652-661.
Williams, M. A., McGlone, F., Abbott, D. F., & Mattingley, J. B. (2005). Differential amygdala response to happy and fearful facial expressions depends on selective attention. Neuroimage, 24, 417-425.
Last Update: 6/8/2016